
The Big Picture
This is your overview of the endeavor of extending healthy life.
We'll answer basic questions about the field: Do we have promising drugs in clinical trials? How many people work on longevity (a thousand? fifty thousand?). And more importantly, we will lay out the system that produces more healthy years of life, and how its productivity could be increased. If you want to dive into the science go here instead.
We're only covering 'true longevity', i.e. science-backed treatments that could change healthy lifespan in normal people and/or eliminate diseases that most people get with age. Longevitainment, i.e. wellness or evergreen health advice rebranded as longevity, and purely cosmetic treatments are not considered.
Let's look at where progress comes from.
The Production Function for Longevity
Though the science of longevity is new, the system that converts it to healthy years of life is the same as for other biomedical research. So let's understand that first.
We have been steadily adding about one year of life per decade, going back more than a hundred years (and not just by reducing childhood mortality, adult lifespan has risen too). For a long time this came from sanitation and later antibiotics reducing infectious disease deaths. But since WWII a large fraction (perhaps a third) has come from effective drugs like insulin and statins. This fraction will need to increase as public health becomes optimized.
Effective therapies go through multiple steps before they start improving our health. They start as therapeutic hypotheses, arising from government- (or sometimes industry-) funded basic research. Some of these are actionable with current technology, and investors (including large biopharma companies) evaluate the scientific and other risks of developing the hypothesis. (Other therapeutic hypotheses need to wait for new technology, e.g. advances in gene delivery or protein design).
The next stage is drug development, where the concept is turned into a physical product that achieves the desired intervention (e.g. removing or delivering a target protein). This is undertaken by fresh biotech companies, takes a number of years (biology is hard!) and millions-tens of millions of dollars.
Products that achieve the desired specs are then tested in (three) stages of human trials for safety and efficacy. Each trial stage is larger and more expensive (millions at first to hundreds of millions). For age-related diseases, the total process takes at least five years. At each stage some of the hypotheses are discarded for a range of reasons. So we need dozens of therapeutic hypotheses for each eventual success.
The longevity field is too young to have contributed to the healthy years added so far. But as infectious disease falls and age-related diseases rise as the reason we die, longevity medicine will be essential to keep the graph of healthy lifespan going up.
Longevity Today
Experimental biology for longevity started in the 1990s and didn't really get going until late 2000s. So it's only barely older than CRISPR (and a lot more complex), and the field is still very small.
Global funding is roughly $1B/yr, a fraction of the ~$400B spent on biomedicine overall. While $1B sounds large, it is roughly what a single city like Boston or Paris spends annually just on Uber rides.
The number of researchers working on longevity is probably between five and ten thousand. For comparison, that's the number of employees at Best Buy or a single mid-large car factory.
As a result, the number of therapeutic hypotheses from the longevity field is still low. We estimate about 5 per year, up from <1 pre-2000. From this we'd expect a single effective drug per decade.
Longevity is still pre-paradigmatic: while we’ve discovered important biology, we lack a consensus 'theory of aging.' We still need to put more puzzle pieces down, and probably move some around.
Another consequence of the field's youth is that hardly any discoveries have had time to go through the process of drug development and clinical trials. And until there's a clear win from the field, both the probability of success and the logistics of navigating trials and reaching patients remain uncertain. This is a key part of why investment remains low.
Altogether, the longevity field *in its current state* might be expected to contribute ~0.01 years of lifespan per decade, starting in the late 2020s.
How would we increase that number?
Inputs to Longevity
What does the longevity field need to grow?
What conditions would produce 0.1 or even 1 year of healthy life every year?
We have to increase the number of therapeutic hypotheses per year.
This needs a combination of more (good) people working on longevity, and increasing what each person can do through funding and tools.
Good people can either be new scientists (which Norn supports through Nexus and Talent Bridge), or scientists from other fields who start working on aging (with Norn supports through Impetus Grants).
While AI guarantees increased leverage in reasoning and analysis, generating the high-quality data to feed these systems will take longer. Brute force can work, but new biotechnologies that change what we can change and measure at scale have at least as much promise.
The first successful longevity drug will trigger a huge increase in funding, as investors realize there's a real path to the largest healthcare market in the world.
This will be amazing for the field. The more talent exists when that happens the better we'll be able to take advantage, without having to wait to train people or fill an empty well of basic research.
Every good person who starts working on longevity today both adds hypotheses that could become the first breakthrough drug, and puts the field in a better place for expansion.
Bottlenecks for Longevity
Without sufficient input the system cannot produce healthy years of life. Once input is in place we will be limited by other bottlenecks. We've already identified the biggest of these:
Reaching that first success in humans is hindered by the duration and cost of trials. The long wait for readouts makes us hesitant to even attempt the experiment. This would change dramatically with a biomarker we trust to predict the future outcomes of trials, and right now the most significant bottleneck is to validate whether any current 'aging biomarker' actually predicts improvements in health from longevity interventions (and if not, produce one). Once something exists, we need to get the FDA on board with using it in trials. Likewise, any improvement to the cost and bureaucracy of running clinical trials will help longevity.
Just like trials, preclinical experiments slow down when we can't measure things that predict future health.
Aging is not a single molecular change, but the loss of coordination between physiological systems. This only happens in living organisms, but we don't have good ways to measure it over time both because time passes slowly and because many things we wish to measure harm the organism.
Beyond slowing down experiments, this makes it harder to determine causal relationships (because we can't tell what happens first) and makes fewer experiments involving aging happen (because they need old animals) even for age-related diseases.
As we increase the pipeline of therapeutic hypotheses, we should also ensure that they are diverse - because the field is preparadigmatic, we should not assume that the most effective path is one we're already on.
Broadening the pipeline would be helped by new ways to deliver genetic therapies. As it is, many genes that change the rate of aging in animals can't be drugged in humans with the types of medicines we have today (except perhaps in gene-edited newborns), and we often can't deliver young cells or organs to an old body.
Increasing the size of the field is good and necessary. Solving the highest leverage bottlenecks in parallel is fantastic, since a clearer path also attracts more people and funding.
Seeing the Future
Once we're thinking deeply about the system that produces longevity, we realize that today’s breakthroughs create tomorrow’s infrastructure and regulatory needs.
We know some of what the future holds, and should start long lead-time projects to prepare:
We know that intelligence will become more abundant within years. So what are the datasets needed for impactful inference about how to treat aging? And what is the 'dark matter' of biology that we don't have good ways to measure?
We know that human trials will be very slow unless we have a surrogate endpoint, and the FDA requirements for approving one. This includes long-term studies of human health comparing the biomarker to health outcomes - could those start today, instead of waiting until they're gating progress?
Once we get really good at solving diseases, we might need to start thinking about which diseases will ail us if we aren't dying from the ones we do today. Could we reach a point of predicting exactly how the body will fail by extrapolating from what cells are doing?
While we accelerate we keep our eye on the big picture, looking ahead to start addressing new bottlenecks before we reach them to avoid losing momentum.
Longevity Industrialized
Norn works to make longevity as impactful as antibiotics.
The longevity field today is artisanal: a small number of experts, trained through apprenticeship, doing research driven by curiosity and what funders will support. This has given us the essential insight that aging is biologically malleable.
To 100x the healthy years we produce we have to industrialize longevity, reinvent the field to allow consistent and efficient problem solving, generating and validating therapeutic hypotheses at a scale that eliminates the element of luck.
By increasing the number and leverage (both intellectual and experimental) of longevity researchers, making therapeutic hypotheses abundant, standardizing and validating how we measure aging so we can test those hypotheses, and formalizing ways to run rapid human trials, we can increase throughput by orders of magnitude.
We've got the blueprint. Let's build the system to extend healthy life.
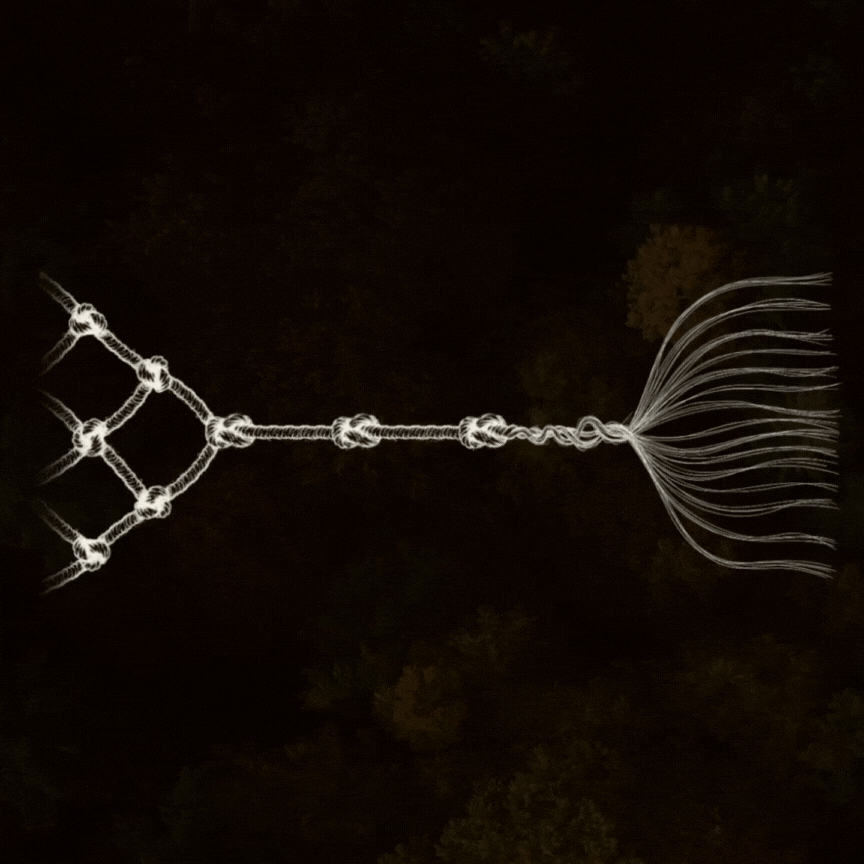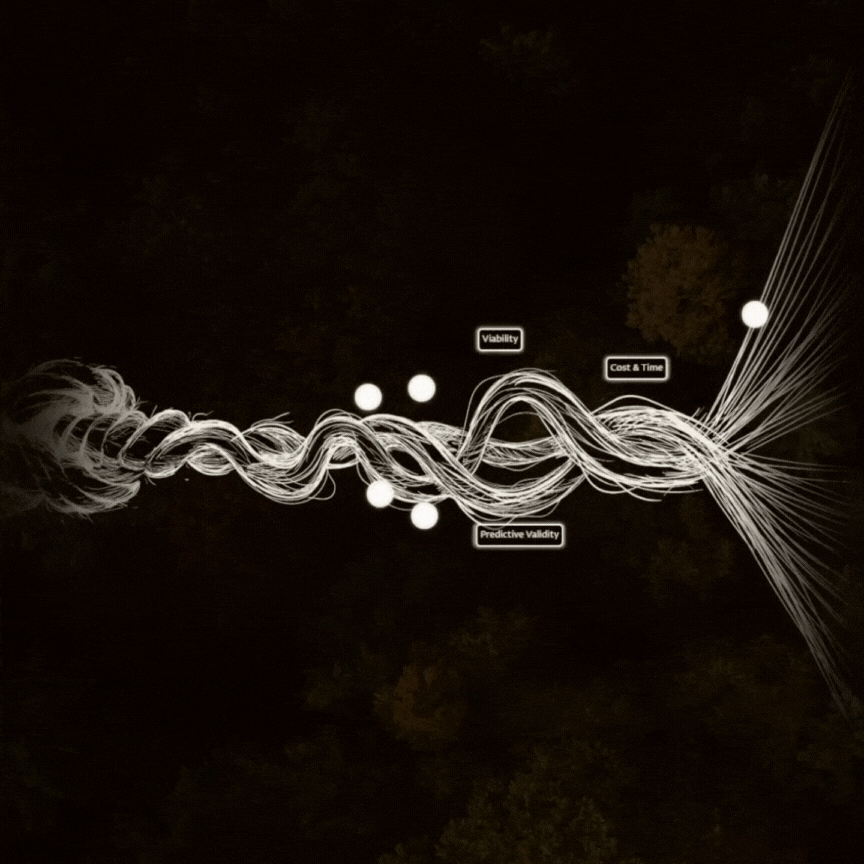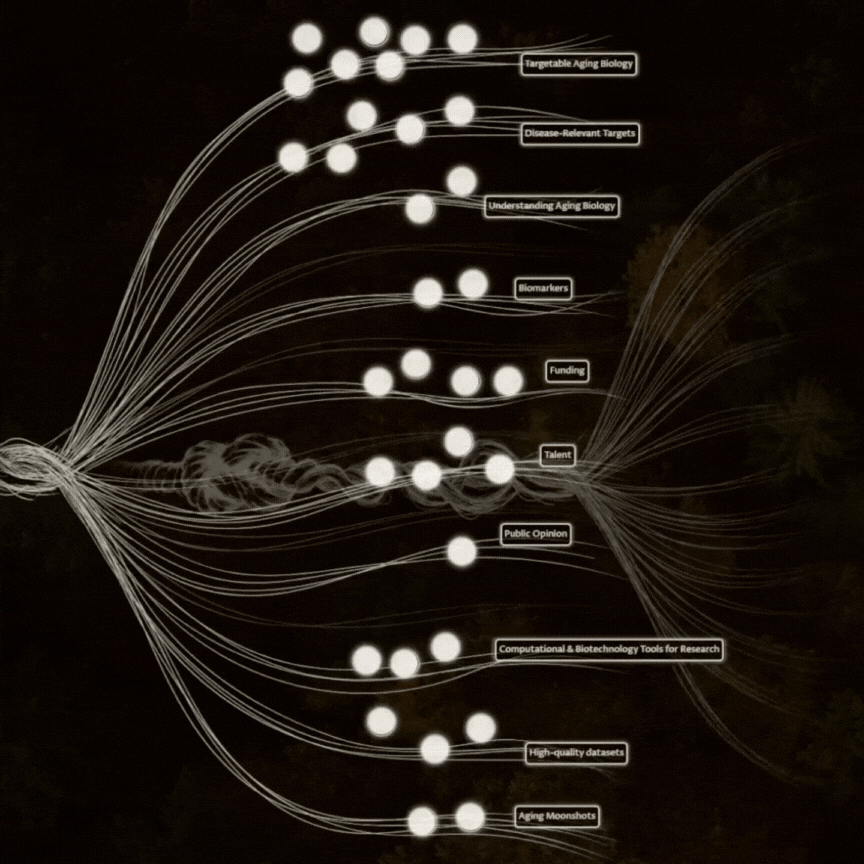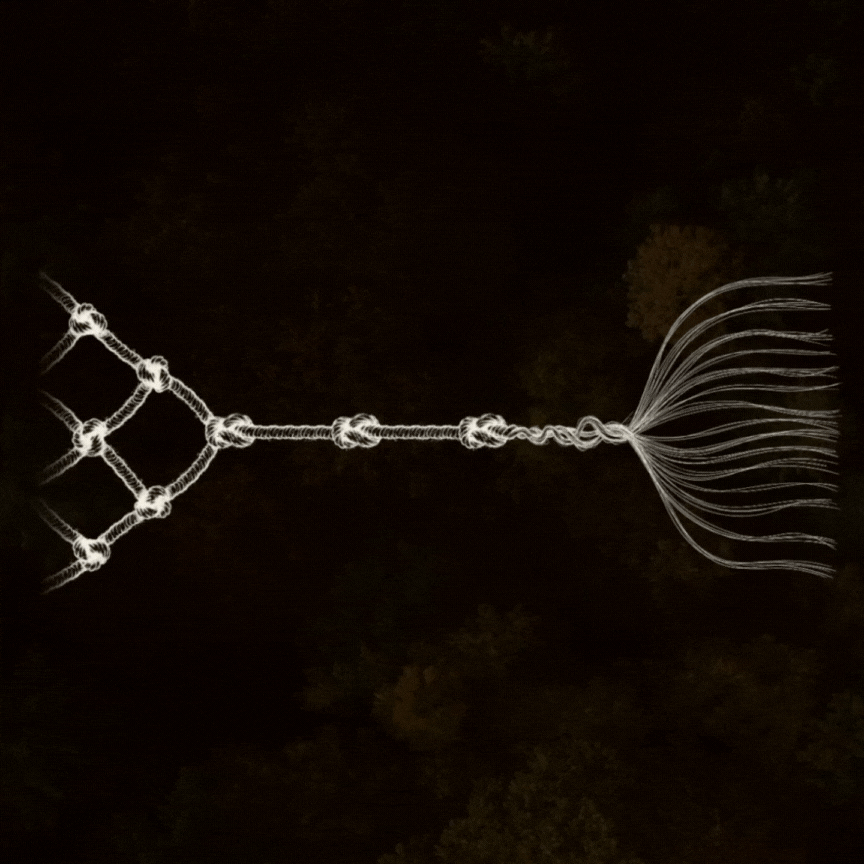
Dive Deeper into the Details
Go deeper on the production function of healthy years, how different factors influence each other, and the most important research, companies, and resources that populate each stage.
